CPAP Machines
Please Login to View Exclusive ResMed Pricing.
CPAP Accessories
Disposable Fine Filter for DreamStation CPAP Machines (6 Pack)
$12.00
Sale!
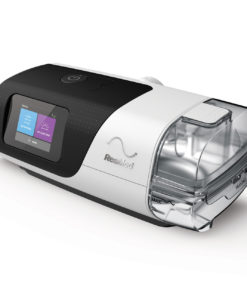
Auto CPAP Machines
Original price was: $1,899.00.$699.00Current price is: $699.00.
Sale!
CPAP Accessories
Original price was: $79.00.$69.00Current price is: $69.00.
CPAP Accessories
$7.00
CPAP Accessories
$10.00
Sale!
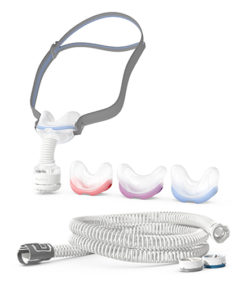
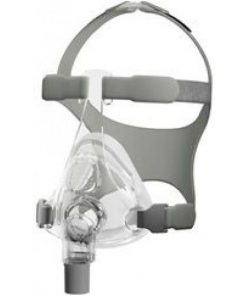
Full Face Masks
Original price was: $180.00.$129.00Current price is: $129.00.
Sale!
CPAP Accessories
Original price was: $59.00.$49.00Current price is: $49.00.
Sale!
Original price was: $240.00.$160.00Current price is: $160.00.